

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 4, No 1:28ó32 © 2004
All rights reserved. Published by Pro Otology Association
The Excitability Test in the Prognosis of Bellís and
Traumatic (Due to Temporal Bone Fracture)
Facial Nerve Palsy
G. Psillas, A. Psifidis, J. Daniilidis
ORL Clinic, Aristotelian University of Thessaloniki, AHEPA Hospital, Thessaloniki, Greece
ABSTRACT
Objectiv: This study assesses the prognostic utility of the excitability test in Bellís and traumatic (due to temporal bone fracture) facial palsy.
Study design: A retrospective case review.
Setting: The study was performed at a university hospital (tertiary referral center).
Patients: 163 patients, 108 patients with Bellís palsy and 55 patients with traumatic facial palsy.
Intervention: All the patients underwent the excitability test. The proportion of patients for each House-Brackmann grade in relation to excitability testís results (normal-diminished-no response) was recorded, until their final facial function outcome.
Results: Good functional outcome was noted in all patients with normal excitability test and poor recovery in patients with no response in all traumatic and in all but two in Bellís palsy. 62% of patients with diminished excitability test returned to normal facial function. The proportion of patients with normal excitability test was gradually increased from House-Brackmann VI to I and with no response reduced from VI to III. Comparing Bellís and traumatic cases regarding recovery over clinical course and excitability test measurements, a more significant ratio of patients with Bellís palsy was found with normal excitability test (p<0.01) and in traumatic cases with no response (p<0.05).
Conclusions: The excitability test is of a high predictive value. It forecasts a complete improvement of facial function when it is normal and a poor recovery when it is completely lost. When diminished it is rather in favor of a satisfactory evolution. Based on the above electrophysiological results, the trauma apparently has a more aggravating effect on facial nerve integrity than other idiopathic processes.
Key words: Facial palsy, Bellís palsy, Temporal bone trauma, Excitability test, Prognosis.
Pro Otology 1:28ó32, 2004
Introduction
Percutaneous electrical stimulation of the facial nerve to assess facial palsy was first described by Duchenne in 1872 (1). Since then several electrophysiologic methods have been developed to evaluate the neural damage following facial nerve palsy. It has been advocated that the motor end-plate can retain its fully excitability for five to ten days during which time the last fragments of axis cylinder in the motor end-plate disappear (2). The excitability of the end-plate then diminishes so that only the muscles fibers remain excitable. Thus, it is important to predict degeneration changes before that period of time in order to adapt the appropriate treatment. In this way electroneurography has been conducted, but its reliability and predictability of facial function recovery have been doubted (3).
In 1963 Laumans introduced the minimal nerve excitability test of the trunk at the stylomastoid foramen. Although a few studies questioned its prognostication value, there are others that supported it, like the nationwide investigation in Japan in 1988 (4-7).
Nevertheless, there has been no study so far to extensively evaluate the excitability test (ET) in relation to currently used worldwide House-Brackmannís grading system, in Bellís and traumatic facial palsy (8).
In this study we assessed the ET by electrophysiologically recording the clinical course of Bellís and traumatic facial palsy due to temporal bone fracture and compared them in order to speculate on the underlying pathological processes.
Materials and Methods
163 patients, aged 8-87 year old, mean 45.7, 71 males and 92 females, were included in this study. These patients were divided into two groups; in the first group there were 108 with Bellís palsy and in the second 55 with traumatic facial palsy following temporal bone fracture. Bellís palsy was defined as an acute and idiopathic facial paralysis without the following: central nervous system disorders, neoplasms, otitis media or herpes zoster oticus. In all the subjects facial palsy was unilateral. Most patients underwent treatment with steroids. Cases in which facial nerve transection had occurred have been excluded.
The ET was performed with the Myoton 2 Facial Nerve Stimulator. The stimulator was powered by a 22.5-volt battery and delivered constant current in 1 ms pulses at a rate of 2 per second. The standardized Cokerís technique was used to conduct ET (9). The testing bipolar probe was applied to the branch to the orbicularis oculi at the lateral rim of the bony orbit and to the branch to the orbicularis oris at the lateral body of the mandible just above the groove formed by the facial artery. The main trunk was not stimulated because it was generally poorly tolerated and presented a relatively important threshold values variation (10).
Current intensity, via the probe, was increased from zero to a level sufficient to generate a regional muscle contraction and then reduced slowly until no twitch could be detected. The current intensity level at which a barely visible muscle twitch was elicited was determined as the nerve excitability threshold first for the normal side and then for the affected one. Comparing the nerve excitability threshold at both sides we obtained the three following cases:
- a difference of excitability of 3.5 mA or less was defined as ďnormalĒ,
- a difference of excitability of 3.5 mA or greater between the two sides was defined as ďdiminishedĒ;
- ďno responseĒ in the ET characterized those to whom no facial reaction was obtained no matter how much current was applied (11).
All the above patients, referred to our center, underwent ET as soon as possible after the third day from the onset of facial palsy, but were tested before the tenth day, in order to assess ETís prognostic value. Follow-up was carried out for at least one year until the maximal recovery at frequent intervals of two days and then of a week and a month. Facial nerve function was assessed according to the House-Brackmann (HB) grading system (I - VI).
Patients with Bellís and traumatic facial palsy were subdivided according to ET results (normal Ėdiminished Ė no response) and HB grade at presentation. The number of patients and their facial function in HB grade were recorded at the final outcome.
Moreover, the proportion of patients in relation to ET results was demonstrated for each HB grade at any time of the clinical course of Bellís and traumatic facial palsy respectively.
Two and five patients with Bellís and traumatic facial palsy respectively underwent decompression surgery, so in these cases the ET was performed only pre-operatively.
Results
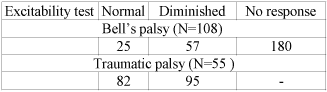
In the first group of patients with Bellís palsy, 75 out of 108 patients had normal ET, 18 diminished and 15 had no response (Table. 1). There were 19 out of 108 patients (17%) with poor outcome (at least HB III) that was not so different from the 16% from Peitersenís report (12), who studied the natural history of Bellís palsy. At presentation, there were 8 patients with HB VI (8/108, 7%) and 62 with HB V (62/108, 57%). When the ET was normal (N=75) all the patients had satisfactory outcome (HB I-II). When the ET was diminished more than half of patients with complete facial paralysis (HB V) achieved HB I-II, (10/16, 62%). From the remaining 15 patients, with no response in the ET, 13 have never returned to normal facial function. However, two patients recovered to HB I (2/15, 13%); the first one, female aged 31 was already in psychotropic therapy before Bellís palsy and the other was an elderly woman aged 78.
In the second group of patients with traumatic facial paralysis, 38 out of 55 (69%) returned to HB I-II, which is close to the 63% of Brodieís study (13). Good functional outcome was noted in all patients with ET normal (N=22) (Table. 2). When the ET was diminished, then 11 patients with HB V (11/16, 69%) had satisfactory facial function. The remaining 5 poorly recovered to HB III. All the patients (N=12) with no response in the ET at the onset of paralysis had poor outcome (III to VI). 11 out of 55 patients (20%) had HB VI, in contrast to 7% of Bellís palsy patients, reflecting the severity of damage in traumatic cases.
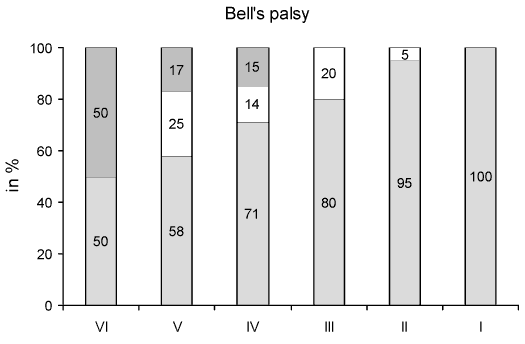
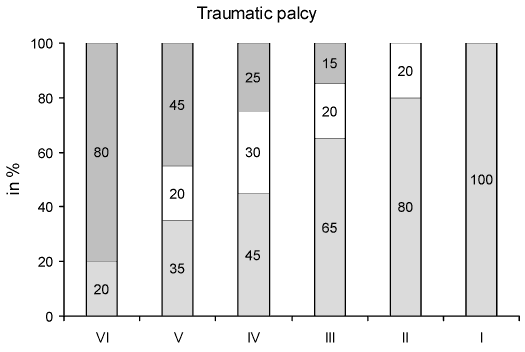
In Bellís and traumatic facial palsy (FIG. 1, FIG. 2), a gradual increase in patients with normal ET was found, in relation to better facial function in the HB grading scale. Thus, in Bellís palsy, the ET was normal in 50% of patients with HB VI, 71% with HB IV and 80% with HB III, while in traumatic palsy the values were significantly lower at a range of 20%, 45% and 65% respectively (p<0.01, t-test). On the other hand, in traumatic facial palsy no response in the ET was found in a significantly higher proportion of patients than in Bellís palsy (p<0.05, t-test), that is in 80% of patients with HB VI, 45% with HB V, 25% with HB IV and 15% with HB III instead of 50%, 17%, 15%, but none with HB III in Bellís palsy respectively. The proportion of cases with diminished ET was similar in Bellís and traumatic facial palsy for HB V, IV and III.
In Bellís and traumatic facial palsy the meantime of recovery to HB I or II was calculated (Table. 3). In patients with traumatic facial palsy to whom the ET was normal and diminished there was a delay of 57 and 38 days respectively compared to Bellís palsy.
|
|
FIG 1. Proportion of patients with Bellís palsy for each House-Brackmann grade (I-VI) over their clinical course according to normal (in blank), diminished (in white) and normal (in gray) excitability test. Note the gradual increase of patients with normal excitability test in relation to better facial function in House-Brackmann grade and the parallel decrease of patients with no response in the test. |
|
|
FIG 2. Proportion of patients with traumatic facial palsy (due to temporal bone fracture) for each House-Brackmann grade (I-VI) over their clinical course according to normal (in blank), diminished (in white) and normal (in gray) excitability test. There is a gradual increase of patients with normal excitability test in relation to better facial function in House-Brackmann scale and decrease of patients with no response in the test (compare with Bellís palsy in FIG.1). |
Discussion
According to our results, in Bellís and traumatic facial palsy, due to temporal bone fracture, the final clinical outcome was directly related with ET results at presentation, which makes ET a reliable prognostication tool. That is, all the patients with normal ET (N=97) achieved a good facial motor function, regardless of facial palsy aetiology. On the other hand, in case of no response in ET, 87% of patients had unfavourable outcome in Bellís palsy and 100% in traumatic palsy. When ET was diminished, 72% of patients (28 out of 39) returned to satisfactory facial function. Thus, ET has a relatively high prognostication value in these most common clinical entities of facial nerve dysfunction.
In other studies, the predictive value of the ET seems to be slightly lower. In Campbellís study 90% of patients with Bellís palsy achieved ďcompleteĒ recovery with ET normal and 80% in Devrieseís (14,16). In the event of no response in the ET in Bellís palsy 80%, 73% and more than 50% had poor recovery (13,14,16). However, comparisons with these studies were difficult to make since HB grading system was not available at that time or was not used.
Regarding traumatic facial paralysis, no study except that of Campbellís referred to the ET thoroughly. There were only few reports with special focus on the management of temporal bone trauma, which mentioned ET results, but the number of subjects was small and facial nerve grading scale was not the same (17-19). So, among 46 patients, 75% with normal ET and 91% with lost excitability had unsatisfactory outcome, which again were somewhat lower than our results (14).
In our sample, diminished ET was better correlated with favorable outcome. In Bellís palsy 62% of patients with diminished ET recovered to satisfactory facial function, compared with 49%, 27% and 25% (14-16). Equally in traumatic facial palsy 69% of our cases returned to facial function HB I-II instead of 40% (2 of 5) (14). It is known that even when the paralysis is complete (HB V or VI), it is always necessary to distinguish neuropraxia or reversible conduction block from axonal degeneration. This distinction is prognostically crucial, since neuropraxia patients usually recover completely without treatment, while patients with complete axonal degeneration experience delayed and poor return of facial function (20). Fisch established 90% axonal degeneration within two weeks after onset of the facial palsy as the critical value for poor prognosis; he postulated that as long as 10% of the facial nerve fibers remain conductive for electrical stimulation, a sufficient number of endoneural tubes remains intact and ensures proper regeneration of the degenerated nerve fibers (21). Thus, normal, even diminished ET represents any potential state of the facial nerve fibers up to 90% of degeneration, each of which is capable of being spared or injured to a different degree at any one time. Consequently, the possibility for any patient with any HB grade to return to normal function is considerable. No response in the ET is apparently associated with total degeneration of the nerve and thus the prognosis is poor.
In Bellís and traumatic facial palsy the proportion of patients with normal ET was gradually increased from HB VI to I (FIG. 1, FIG. 2). Conversely, the proportion of patients with no response in the ET decreased passing from HB VI to III. This validates the ET, which can reflect the functional state of facial nerve in each corresponding HB grade. The transition of the clinical facial function from a worse to a better HB grade is reasonably associated with increased stimulable facial nerve fibers. In other words, there is a higher chance for a normal ET in HB III than HB VI.
| Table 3. Mean time recovery (in days) to HB I or II of patients with Bell's and traumatic palsy regarding excitability test results (no patients recovered to H.B. I or II). |
|
The proportion of patients with normal ET was found significantly higher in Bellís palsy than in traumatic palsy for each HB grade respectively. Similarly the proportion of patients with no response in ET was significantly higher in traumatic than in Bellís palsy. Taking into account this divergence in proportions we can assume, through ET results, that the damage to the facial nerve in traumatic facial palsy due to temporal bone fracture is more frequent and considerable than in Bellís palsy. This was also confirmed by the fact that the mean time recovery to a satisfactory facial function takes longer in traumatic cases than in Bellís palsy with respect to ET results (Table. 3); there was not even recovery in traumatic cases with no response in the ET. In both, Bellís and traumatic facial palsy the site of injury is generally the same, located in the perigeniculate, labyrinthine and meatal segment of the facial nerve and results in retrograde axonal degeneration (22,23). However, in traumatic cases the pathogenic pressure is exerted on the nerve from without rather than from within the intraneural space. It is possible that following the direct facial nerve injury due to temporal bone trauma an ischemic process is occurred, which is not encountered in Bellís palsy according to other studies (24-26). This ischemia results in secondary edema, which causes increased pressure within the closed space of the fallopian canal. Then a delayed injury of the nerve is produced, which inevitably aggravates the damage of the facial nerve (24).
In conclusion, the ET is of a high predictive value in Bellís and traumatic facial palsy due to temporal bone fracture and forecasts a complete improvement of facial function when it is normal and a poor recovery when it is completely lost. A diminished ET is rather in favor of a satisfactory evolution or in the worst of cases of a recovery to HB III. It can be used reliably in the assessment of the above diseases of the facial nerve and be consistently implicated in the judgment of surgical intervention.
REFERENCES
Duchenne GB. De lí electrication localisťe. Third Edition., Paris: Baillere, 1872.
Adams RD, Denny-Brown D, Pearson CM. Diseases of muscle London: Cassell, 1953.
Sittel C, Guntinas-Lichius O, Streppel M, et al. Variability of repeated facial nerve electroneurography in healthy subjects. Laryngoscope 1998;108:1177-80.
Laumans EPJ, Jongkees LBW. On the prognosis of peripheral facial paralysis of endotemporal origin. Part II. Electrical tests. Ann Otol Rhinol Laryngol 1963;72:621-36.
May M. Nerve excitability test in facial palsy: Limitations in its use, based on a study of 130 cases. Laryngoscope 1972;82:2122-8.
6. Kraus P. Reliability of the nerve excitability test in Bellís palsy. J Laryngol Otol 1970;84:719-22.Koike Y, Aoyagi M, Ichige A. Nationwide investigation on diagnostic methods for facial palsy. Acta Otolaryngol Suppl (Stockh) 1988;446:30-5.
House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985;93:146-7.
Coker NJ, Fordice JO, Moore S. Correlation of the nerve excitability test and electroneurography in acute facial paralysis. Am J Otol 1992;13:127-33.
Lewis BI, Adour KK, Kahn JM, et al. Hilger facial nerve stimulator: a 25-year update. Laryngoscope 1991;101:71-4.
Kumar A, Applebaum EL. Evaluation of the facial nerve. In: Nadol JB, Schuknecht HF, eds. Surgery of the ear and temporal bone. New York: Raven Press, 1993:71-7.
Peitersen E. The natural history of Bellís palsy. Am J Otol 1982;4:107-11.
Brodie HA, Thompson TC. Management of complications from 820 temporal bone fractures. Am J Otol 1997;18:188-97.
Campbell EDR, Hickey RP, Nixon KH, Richardson AT. Value of nerve-excitability measurements in prognosis of facial palsy. Br Med J (Clin Res) 1962;2:7-10.
Leclaire R, Tremblay L, Dupuis M. Prognostic value of nerve excitability test in Bellís palsy. Can J Otolaryngol 1975;4:352-7.
Devriese PP, Schumacher T, Scheide A, et al. Incidence, prognosis and recovery of Bellís palsy. A survey of about 1000 patients (1974-1983). Clin Otolaryngol 1990;15:15-27.
Adour KK, Boyajian JA, Kahn ZM, et al. Surgical and nonsurgical management of facial paralysis following closed head injury. Laryngoscope 1977;87:380-90.
Griffin JE, Altenau MM, Schaefer SD. Bilateral longitudinal temporal bone fracture: a retrospective review of seventeen cases. Laryngoscope 1979;89:1432-5.
McKennan KX, Chole RA. Facial paralysis in temporal bone trauma. Am J Otol 1992;13 :167-72.
Collier J. Electrodiagnosis and surgical indications in facial palsy. Arch Otolaryngol 1963;78:421-6.
Fisch U. Surgery for Bellís palsy. Arch Otolaryngol 1981;107:1-11.
Fisch U. Facial paralysis in fracture of the petrous bone. Laryngoscope 1974;84:2141-54.
Felix H, By DL, Fisch U. New aspects of facial nerve pathology and temporal bone fractures. Acta Otolaryngol 1991;111:332-6.
Chang CYJ, Cass SP. Management of facial nerve injury due to temporal bone trauma. Am J Otol 1999;20:96-114.
Liston SL, Kleid S. Histopathology of Bellís palsy. Laryngoscope 1989;99:23-6.
Fisch U, Felix H. On the pathogenesis of Bellís palsy. Acta Otolaryngol 1983;95:532-8.
|
Pro Otology |
Journal Home Contents Preview Next |