

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 4, No 1:33—36 © 2004
All rights reserved. Published by Pro Otology Association
Postnatal Development of the Spiral Ganglion in Rats
Emilian Ivanov, Konstantin Koitschev
Department of Anatomy, Histology and Cytology, Medical University, Pleven, Bulgaria
ABSTRACT
Hypothesis: The aim of the present study is the peculiarities in the growth and the ultrastructure of the cells in the spiral ganglion in the white rat in early postnatal period.
Background: Contradictional data about the type 1 and type 2 neurons origin in the different vertebrate sustain the scientific interest to the embryonal development of the spiral ganglion. There are essential differences in the structure of the cell bodies and the distribution of their central and peripheral processes. Both form two separate afferent systems for the inner and outer hair cells which suppose their different roles in the processing the auditory information
Methods: The white rat spiral ganglion development during first two weeks after birth was investigated by morphometric and ultrastructural methods. Thirty-two rats were used, divided into 9 age groups. After decapitation, the cochlear were fixed, decalcified (for various times), embedded in durkupan (details in the text bellow). Ultrathin sections were double contrasted and observed with a Tesla 500 BS electron microscope. Thick sections were cut and stained with 1% toluidine blue for examination with Olympus System Microscope BX 51. Critical periods about cytoplasmic organization and myelinization of perikaryons and neurities of both types of neurons were considered. In small amount of type 2 neurons were registrated axosomatic synapses whose origination and physiological function are discussed.
Results: During first week neurons were characterized by homogenous structure and growing activity and eventually reached the size of adults. A part of this undifferentiated population dies by apoptosis. During the second week neurons were differentiated in type 1 and type 2. Critical periods about cytoplasmic organization and myelinization of perikaryons and neurities of both types of neurons were considered. In small amount of type 2 neurons were registrated axosomatic synapses which origination and physiological function are discussed.
Key words: Spiarl ganglion cell, Development, Apoptosis, Axosomatic synapses.
Pro Otology 1:33—36, 2004
Introduction
Contradictional data about the type 1 and type 2 neurons origin in the different vertebrate sustain the scientific interest to the embryonal development of the spiral ganglion. There are essential differences in the structure of the cell bodies and the distribution of their central and peripheral processes. Both form two separate afferent systems for the inner and outer hair cells which suppose their different roles in the processing the auditory information (4). Ultrastructural researches have classified other subpopulations (type 2A, type 3 and type 4) whose functions remain in still hypothetic (6,9). The growth, migration, the programmed cell death, the myelinization and synaptogenesis vary among the different species of vertebrates (10,11). These processes are managed by genes the expression of their products is characterized by different constellation and supplement the morphological pattern of spiral ganglion development (1).
The aim of the present study is the peculiarities in the growth and the ultrastructure of the cells in the spiral ganglion in the white rat in early postnatal period.
Material and methods
Thirty-two rats were used, divided into 9 age groups: new-born – 2,4,6,8,10,12,14 and 16 days old. After decapitation, the cochlear were fixed for 2 h in a combined solution of 1.25% glutaraldehyde, 1% paraformaldehyde and 0.015% CaCl2, in 0.1 M cacodylate buffer (pH 7.2-7.4). After that they were decalcified in 2% EDTA and 0.2 M sodium cacodylate (pH 7.2) for various times ranging from overning to 2 weeks with frequent changes of the decalcifying solution. The material completed fixation in 1% OSO4 for 1 h and embedded in durkupan. Ultrathin sections were double contrasted with 4% uranilacetate and 0.1% lead citrate and were observed with a Tesla 500 BS electron microscope. Thick sections were cut and stained with 1% toluidine blue for examination with Olympus System Microscope BX 51.
Results
At day 1-3 after birth the spiral ganglion is built of dense packed neurons and Schwann cells. The neurons are homogenous population of spherical and polygonal cells covered by single layer of glial cell processes. In their cell organization the dominant structure are nuclei with prominent nucleoles; the cytoplasm is rich of polysomes, Golgy complexes, vacuoles and mitochondria. The cell membrane frequently forms invaginations and pinocytotic vesicles (FIG. 5, FIG. 6). Average cell size was (x = 155.06 ± 23.5 µm2) (FIG. 1).
|
|
|||||
|
|
The Schwann cells are characterized with smaller size and more osmiophilic cytoplasm in comparison with the neurons. Their nuclei are heterochromatic and irregular in shape surrounded by halo of granular cytoplasm. Schwann cells processes are wedged among the neurons and ensheet the perikaryon and neurities. Sometimes more than one Schwann cell participates in the glial covering of a neuron. In this period mitotic division of Schwann cell precursors is observed (FIG. 7).
Between 4-7 postnatal day neurons of the spiral ganglion are still homogenous population with the traces of immaturation. Their ultrastructure is close to these from the previous period. The cells however are bigger in size in comparison with the first period and not that closely packed in side the ganglion. x = 197.24 ± 24.7 µm2 (FIG. 2). Some amount of them dyes with typical signs of apoptosis – chromatin condensation and cell nucleus shrinking (FIG. 8).
|
|
The same programmed cell death of the spiral ganglion neurons is seen only in day 5-7 after birth, but not in the material of grown animals. Schwann cells in this period have finished their terminal mitosis. Their processes form from 2 to 4 layers around some perikaryons and in others they are still in one layer. Even in the same site of the cochlea the ganglion cells myelinization is at a different level.
|
|
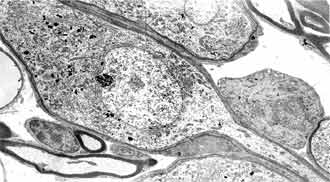
FIG 11. Type 1 and type 2 neurons 20PN x 4 000. |
In the period of 8-16 day after white rat birth the neuronal population is differentiated in type 1 and type 2. Maturity of the neurons of the basal coil is reached earliest. The neurons of type 2 are differentiated first in 8 postnatal day. They are smaller in size, grouped, pseudounipolar, ensheeted only by Schwann cells. Their nuclei present deep nucleoplasmic invaginations with fine chromatin, piled up on the periphery. The cytoplasm contains big amount of neurofilament bundles and scanty amount of other organelles (FIG. 9). They are characterized by bipolar bodies, ensheeted with several layers of loose and dense myelin and cytoplasm, rich in granular reticulum, mitochondria and Golgi complexes. The most impressing difference between the two types of neurons is the quantity of the granular endoplasmatic reticulum (FIG. 11). In the cells of type 2 it is scanty, while in those of type 1 it is abundant. The myelinization has progressed. As a rule the myelinization of the neurites takes part before the one of the cell bodies.
In small number of type 2 neurons we found out synaptic terminals pressed between the cytoplasmatic membrane and glial layer (FIG. 10). These terminals are characterized by two types of synaptic vesicles: round with dense centre and oval with light contains. The histogram of the two neuronal types changes quite between 8-16 postnatal days. On 9 postnatal day the histogram keeps the characteristics of the earlier levels of our research, although the two neuronal types are differentiated (xT1 = 216.42 ± 21.66 µm2; xT2 = 130.85 ± 17.08 µm2) (FIG. 3).
On 16 postnatal day the distribution of the maximal sectional area is bimodal, as in adults (xT1 = 228.07 ± 26.88 µm2; xT2 = 136.10 ± 16.40 µm2) (FIG. 4).
Discussion
Structure changes in the white rat spiral ganglion in early postnatal period concerning growth dynamic and changes in the neurons and Schwann cells organization were investigated. On the first postnatal week neurons of the spiral ganglion remain undifferentiated and only growth activity can be observed.
The nucleolar reaction, higher nucleoplasmatic index and the abundance of polysomes is a sign of an enhanced protein synthesis. Comparison between 2 and 6 postnatal day histograms show intense increase of neuronal perikaryons, that reaches the size of matured (neurons) even before differentiation in type I and type II. Ganglion maturation in other animals has shown similar features (9-13).
Programmed cell death (apoptosis) of the part of neuroganglion cells was seen parallely with the growth changes in this period. Many of known investigations in the spiral ganglion development report about apoptosis in the prenatal period but never after birth. Even lately Bax-positive or neurons with fragmented DNA after birth were discovered by using immunohistochemistry and TUNEL methods (2,7,8,12,14).
Ganglion cell apoptosis coincides in time with the outer hair cells afferent processes regression and their efferent processes replacement. Changes in outer hair cells occur because of decreasing production of neurotrophic factors. This is the reason why neurons being in synaptic interaction with them die or differentiate in type II.
Morphological and electrophysiological spiral ganglion maturation follows that is typical of the cochlea basoapical gradient (15). Histograms of 9 to 16 postnatal day exhibit insignificant growth of the neurons. Typical features at the moment are differentiation and myelinization of neurons. We have descripted type II neurons with axosomatic terminals in that correspond to the neurons type 2A in primates and human (6,13). The existence of these axosomatic ends in type II neurons in postnatal rats show that they are not only limited to higher mammals (3). The origin is not determined yet, but most of investigators consider that they originated from the superior medial superior olivary complex. There are only speculations about the physiological role of the type II synapses. Their existence presume additional possibility that efferent olivocochlear bundle could modify the presynaptic afferent activity of the type II neurons on the spiral ganglion level.
REFERENCES
Bard Y-A .Trophic factors and neuronal survival. Neuron 1989;2:1525-34.
Gavrieli Y, Sherman Y, Ben-sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493-501.
Ivanov E, Koitchev K, Cazals Y, Aran J-M. Axo-somatic contacts in the postnatal developing white rat spiral ganglion. Acta Otolaryngol. 1992;112:985-90.
Kiang NYS, Rho JM, Northrop CC. Hair cell inervation by spiral ganglion cells in adult cats. Science 1982;217:175-7.
Kim DO. Functional roles of the inner and outer hair cell subsystems in the cochlea and brainstem. In: Berlin Ed. Recents Advances: Hearing Sci. San Diego, CA: College-Hill Press, 1984:241-62.
Kimura, RS, Bongiorno CL, Inerson NA. Synapses and ephapses in the spiral ganglion. Acta Otolaryngol 1987;Suppl.438:3-18.
Nikolic P, Jarlebark LE, Billett TE, Thorne PR. Apoptosis in the developing rat cochlea and its related structures. Dev Brain Res 2000;119:75-83.
Nishizaki K, Anniko M, Orita Y, et al. Programmed cell death in the developing epithelium of the mouse inner ear. Acta Otolaryngol (Stockh) 1998;118:96-100.
Romand R, Romand MR, Mulle C and Marty R. Early stages of myelination of spiral ganglion cells in kitten during development. Acta Otolaryngol 1980;90:391-7.
Romand R, Romand MR. Myelination kinetics of spiral ganglion cell in kitten. J Comp Neurol 1982;204:81-5.
Schwartz AM, Parakkal M and Gulley RL. Postnatal development of Spiral Ganglion Cells in the Rat. The American Journal of Anatomy 1983;167:33-41.
Sugimoto T, Xiao C, Ichikava H. Postnatal changes in Bax-immunoreactivity and apoptosis of the rat trigeminal primary neurons. Neurosci Lett 1998;258:97-100.
Thiers FA, Burgess BJ, Nadol Jr JB. Prevalence and ultrastructural morphology of axosomatic synapses on spiral ganglion cells in humans of different ages. Hear Res 2000;150:119-31.
Wang Z, Li H, Chi F, et al. Transient Bax-protein Immunoreactivity Prior to Apoptosis of Spiral Ganglion Neurons in the Postnatal Rat. Acta Otolaryngol 2001;121:777-80.
Uziel A, Romand R, Marot M. Development of cochlear potential in rats. Audiology 1981;20:89-100.
|
Pro Otology |
Journal Home Contents Preview Next |