

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 3, No 2—3:123—128 © 2003
All rights reserved. Published by Pro Otology Association
Antibiotic Update - Augmentin Bid for the Treatment of Acute Otitis Media in Children and Adults
*P. Dyakov, †I. Jovchev, *St. Stoyanov, *L. Popov, *T. Karchev
*Department of Otorhinolaryngology, University Hospital “Tzaritza Joanna”, Sofia, Bulgaria
†Department of Otorhinolaryngology, Medical University, Plovdiv
ABSTRACT
Objective: To evaluate the clinical and bacteriologic efficacy and establish the resistance toward a new formulation of Augmentin prescribed twice daily.
Study design: Open label, multicenter study of 60 children and adults with acute otitis media.
Results: All bacterial pathogens recovered from children with AOM were susceptible to Augmentin (100%). This applies to Penicillin resistant S. aureus and S. pneumoniae. In adults clinical improvement was noted in all patients, cure rate reaches 96% (29 cases). Resistance to PEN and AMP is much more limited compared to pathogens isolated from children. In children about 50% of isolated S. pneumoniae is resistant to PEN (intermediate included), while in adults this percent drops below 30.
Conclusion: The new twice-daily combination is excellent for treating AOM caused by penicillin-intermediate and -resistant S. pneumoniae. Augmentin bid can be considered a treatment of first choice for paediatric and adult AOM where participation of resistant bacteria is suspected.
Key words: Augmentin, amoxicillin/clavulanic acid, resistant S. pneumoniae.
Pro Otology 2-3: 123-128, 2003
INTRODUCTION:
Acute otitis media is one of the most frequent diseases affecting children population, yet it’s not uncommon in adults. Bacterial resistance to antibiotics is a growing problem, which is rapidly developing into a major public health concern. Although acquired resistance is a naturally occurring phenomenon, evidence suggests that antibiotic usage and abuse promotes the selection of resistant strains. The management of acute otitis media is complicated by the emergence of resistance to beta-lactam and other antibiotics among common pathogens. This affects mainly the older generation of penicillins and therefore a modern approach, actualising the first-choice empirical treatment in Bulgarian population is needed. In order to reduce the rate of failure, it has been necessary to both increase the dose of penicillin to overcome the reduced susceptibility of Pneumococci and to prescribe a beta-lactamase inhibitor because of the frequent isolation of beta-lactamase producing Hemophilus influenzae and Moraxella catarrhalis. Studies show that the only antibiotic that reaches considerable (respectively adequate) MIC90 against the majority of common upper respiratory tract pathogens acquired in society is Augmentin (amoxicillin/clavulanic acid). (1,2)
METHODS
Primary objectives
The first primary objective of our study is to evaluate the clinical and bacteriologic efficacy of a new formulation of Augmentin (tablets 1g and suspension 457/5ml) prescribed twice daily and providing an increased ratio amoxicillin/clavulanic acid (7:1). The standard three-times-daily formulation has a ratio of 4:1 A/C, consequently the new one offers a high-dose beta-lactam treatment, developed with the aim of providing better coverage for penicillin-resistant strains including Streptococcus pneumoniae, beta-lactamase producing Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus.
Second, the resistance towards the new formulation of Augmentin is also to be evaluated amongst the isolated pathogens, giving a better view of the current bacteriological prevalence in the Bulgarian population.
Secondary objectives
The profile of drug safety will be established based on the adverse reactions that may occur during the active treatment phase.
Study design
This is an open label, multicenter study that was carried out from October 2002 to March 2003 at the University Clinics of Otorhinolaryngology in Sofia and Plovdiv.
Patients:
Patients were included in the investigation if they fulfilled all of the following criteria:
1. Children (aged between 0 and 12 years or having a body weight less then 40kg) and adults (age over 12 or weight over 40kg). (We had 1 child, who was included in the adults group because of his overweight, exceeding 40kg and therefore received adult-dose treatment.)
2. Patients with a clinically proven diagnosis of acute otitis media.
Patients were not included in the case of (note: any of those apply):
1. Known or clinically proven history of hypersensitivity or allergy to Augmentin, penicillins or clavulanic acid.
2. Patients who had a serious adverse drug reaction, proven to be a result of Augmentin treatment or intake.
3. Patients who received any other antibiotic within 4 days of inclusion in this study.
The quota for adults and children was equal between the centres and represented 15 children and 15 adults per centre, resulting in an overall number of 60 patients for the whole investigation. All patients were informed about the drug actions, possible side effects and risks of treatment or manipulations, adverse reactions, the need of follow up visits and microbiological samples. Consent from all patients (or his/her parents in the case the patient was a child) was taken for good cooperation and compliance with treatment.
Adults were treated using the newly registered for use in Bulgaria Augmentin Bid tablets 1g, while children received the new 457/5ml oral suspension. Both were subject to twice daily dosing: adults 2g/24h and children 45mg/kg/24h. Drug treatment continued for 7 days.
This new, high dose formulation provides an increased ratio A/C to 7:1 in favour of Amoxicillin, compared to the classical preparation with a ratio A/C of only 4:1. (In the 375mg tablets this ratio is as low as 2:1).
All patients were advised to take medication during meals, preferably at the beginning, to minimize possible gastro-intestinal side effects and improve absorption.
|
|
Patients were evaluated before the beginning of therapy, during therapy (Days 3-4) and at the end of therapy (Days 7-10). Further down the 3 scheduled visits are described in detail:
1. Visit 1 – before beginning of therapy and on the first day of treatment. Patients were taken their history; local and general status established (see note 1), nasopharyngeal or ear (see note 2) samples were obtained for culture before starting therapy.
Note 1.: Status parameters were observed as follows: general: fever, pain (measured subjectively by the patient on a 5 grade scale); local: tympanic membrane –inflammation, swelling, mobility, colour, bulge, presence of spontaneous perforation and discharge from the tympanic cavity, type of discharge and duration of membrane perforation (for this study patients with spontaneous perforations younger than 12h were preferred). In cases of extreme membrane bulging, a single tympanocentesis was performed and patients were included in the otorrhea group.
Note 2.: Microbiological samples were taken from the ear if otorrhea was present or the latter was released by the tympanocentesis. In the rest of the cases swaps were taken from the nasopharynx.
2. Visit 2 – at mid-therapy. Status was evaluated in the same manner as at the first visit, identical parameters were monitored. In cases with no clinical improvement a new culture was obtained to diagnose a possible resistant strain. Same applied to patients whose first swaps remained sterile or became contaminated.
Identification and susceptibility testing of AOM pathogens were performed by routine clinical laboratory methods.
3. Visit 3 – at the end of therapy/early post therapy
|
|
Status was evaluated in the same manner as at the first and second visits, identical parameters were monitored. The efficacy parameters used were fever and pain clearance, local inflammation symptom clearance, otorrhea clearance and cure rate after 7 days of treatment.
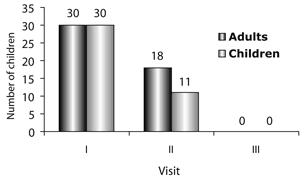
Schematically the study design is represented on FIG. 1.
RESULTS
For the period of this study we treated a total of 60 patients, 30 children and 30 adults. Our youngest patient was 7 months of age, while the older one was 68 years of age. Males were predominant in both age groups. Detailed demographical data for all patients are presented on Table 1.
Clinical results will be reported separately for adults and children because of the major differences in these two groups and the vast diversity of clinical and bacteriological findings.
Children
The clinical signs and symptoms monitored throughout this study showed the following dynamics at the three visits (FIG. 2, FIG. 3).
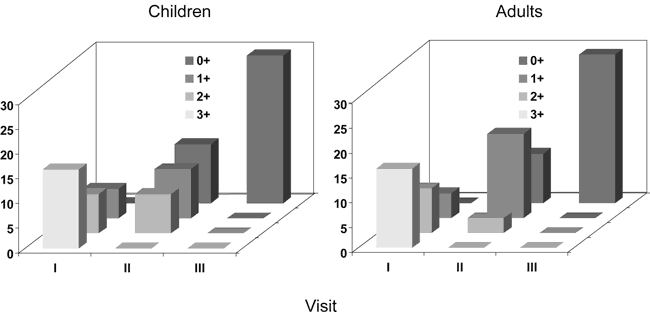
In all patients with noticeable bulging and purulent middle ear contents a single paracentesis was performed and consequently these were included in the perforation present group. Patients with otoscopic signs of middle ear inflammation, but without manifest bulging we assumed the presence of serous exudate (Table 2, FIG. 4, Table 3, Table 4, Table 5).
All bacterial pathogens recovered from children with AOM were susceptible to Augmentin (100%). In other words Augmentin managed to eradicate or clinically cure 100% of children with AOM. (cure rate of 100%). This applies to Penicillin resistant S. aureus and S. pneumoniae (Table 6).
Adults
The clinical signs and symptoms monitored throughout this study showed the following dynamics at the three visits (FIG. 2, FIG. 4).
In all patients with noticeable bulging and purulent middle ear contents a single paracentesis was performed and consequently these were included in the perforation present group. Patients with otoscopic signs of middle ear inflammation, but without manifest bulging we assumed the presence of serous exudate ( Table 7, Table 8, FIG. 5, Table 9).
Clinical improvement was noted in all patients, cure rate reaches 96% (29 cases). All bacterial pathogens recovered from adults with AOM were susceptible to Augmentin, except one isolate of P. aeruginosa. The rest of the bacteria show susceptibility to the common antibiotics that corresponds to expected values, yet resistance to PEN and AMP is much more limited compared to pathogens isolated from children. In children about 50% of isolated S. pneumoniae is resistant to PEN (intermediate included), while in adults this percent drops below 30. Same applies to S. aureus but Klebsiella sp. remains definitely resistant to AMP (100%).
In the case where P. aeruginosa was isolated, therapy with Augmentin continued for the time predefined by the protocol and surprisingly there was clinical improvement, unfortunately minor one. After the course was completed medication continued with another per oral antibiotic concomitantly with a topical solution which led to successful outcome for the patient.
The following table is presented for comparative purposes only and these data ARE NOT a result of this investigation (Table 10).
Treatment was well tolerated, with adverse events being of a mild-moderate and transient nature. The only treatment-related adverse event was diarrhoea, occurring in 2 children (3%) of patients.
|
|
FIG 5. Local signs of acute inflammation (includes: marked erythema, reddish of scarlet membrane, bulging, limited mobility) distributed by visit. |
Discussion
We present some selected data in the literature regarding AOM in children and adults in order to have a better understanding of the distribution, frequency, microbiology and treatment of this pathology.
Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis remain the leading pathogens of AOM (3,4).
Not surprisingly in our investigation among the most common pathogens appears Staphylococcus aureus isolated in 22% of patients, yet the leader stays Streptococcus pneumoniae with 37% of isolates. Fortunately all strains of S. aureus isolated remain susceptible to Augmentin, leaving treatment still one step ahead of bacterial poly resistance.
A recent large, international study conducted by Jacobs, Dagan et al.(5) identified pathogens and susceptibility patterns of AOM in 917 children from Bulgaria, the Czech Republic, Hungary, Romania, Slovakia, Israel, and the United States. Pathogens were isolated from 62% of the patients (in our study we manages to recover pathogens form 80% of the patients). The composite susceptibilities of S. pneumoniae (30% of the patients), untypeable H. influenzae and M. catarrhalis (4%) to amoxicillin-clavulanate ranged from 90% in Israel to 95% in Eastern and Central Europe. The prevalence of resistant S. pneumoniae was highest in patients less than 12 months of age which corresponds to our findings of increased resistance of S. pneumoniae in children. We assume this is a direct consequence of the vast misuse of antibiotics in children, especially by general practitioners. This applies not just to the antibiotic of first choice but to treatment duration as well. Administration of Augmentin for 5 days appears not to provide equivalent efficacy compared to a 7 or 10 day regimen, especially in young children (6,7).
A study by a Japanese group reveals that 46.7% of S. pneumoniae (in our study 50%) isolated from nasopharyngeal swab were drug resistant and they are increasing year by year (4).
The literature confirms that augmentin therapy eradicates or suppresses all strains of S. pneumoniae susceptible to penicillin, and most of strains with intermediate resistance, and strains resistant to penicillin (8)
The new formulation prescribed twice daily guarantees a successful bacteriological response at the end of therapy in most cases. Clinical success rate varies, but is no less than 80%, sometimes reaching values above 98% (2,9,10,11).
Dosing twice daily significantly increases patient compliance over a 7-10 day treatment period and shows equivalent or better clinical efficacy, as well as improved tolerability compared to a t.i.d. regimen. B.i.d. formulation is especially efficient against drug-resistant S. pmeumoniae (11,12).
Most studies indicate that Augmentin is generally well tolerated. A low total incidence of adverse events (3.6%) and no serious events were reported from a large paediatric post marketing study. (9,10,11) The most frequently reported adverse events in children are mild gastrointestinal disturbances. Diarrhoea is generally less frequent with twice-daily than with three-times-daily treatment.
CONCLUSION
Augmentin is a well established broad-spectrum antibacterial treatment which is effective and well tolerated in the treatment of AOM in both paediatric and adult patients. The new twice-daily, high-dose combination proves valuable in treating AOM caused by penicillin-intermediate and -resistant S. pneumoniae. Based on recent recommendations and the available data, high-dose amoxicillin/clavulanic acid (Augmentin bid) can be considered a treatment of choice for recurrent or persistent paediatric and adult AOM where involvement of resistant pathogens is suspected.
In conclusion, this study confirms that b.i.d. Augmentin has the potential to improve patient compliance and so reduce the rate of clinical failure. Other benefits include fewer side effects, improved patient compliance and lower treatment costs.
|
|
FIG 4. Pain subjective scale and presence of pain by visit. |
REFERENCES
Leibovitz E, Dagan R. Antibiotic treatment for acute otitis media.
Int J Antimic Agents 2000;15 169-77.
Dagan R, Hoberman A, Johnson C, Leibovitz EL. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr Infect Dis J 2001; 20(9):829-37.
Block SL, Hedrick JA, Tyler RD, Smith RA, Harrison CJ. Microbiology of acute otitis media recently treated with aminopenicillins. Pediatr Infect Dis J 2001; 20(11):1017-2.
Sugita R, Harada S, Deguchi K, Fujimaki Y, Naito M, Komatsu N. A clinicobacteriologic study on clavulanic acid/amoxicillin in pediatric acute otitis media. Jpn J Antibiot 1999; 52(10):595-612.
Jacobs MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial-resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrob Agents Chemother 1998; 42(3):589-95.
Hoberman A, Paradise JL, Burch DJ, Valinski WA. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatr Infect Dis J 1997; 16(5):463-70.
Cohen R, Levy C, Boucherat M, Langue J, de La Rocque F. A multicenter, randomized, double-blind trial of 5 versus 10 days of antibiotic therapy for acute otitis media in young children. J Pediatr 1998; 133(5):634-9.
Ghaffar F, Muniz LS, Katz K, Reynolds J. Effects of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children with acute otitis media. Clin Infect Dis 2000; 31(4):875-80. 9. Damrikarnlert L, Jauregui AC, Kzadri M. Efficacy and safety of amoxycillin/clavulanate (Augmentin) twice daily versus three times daily in the treatment of acute otitis media in children. The Augmentin 454 Study Group. J Chemother 2000; 12(1):79-87.
Easton J, Noble S, Perry CM. Amoxicillin/clavulanic acid: a review of its use in the management of paediatric patients with acute otitis media. Drugs 2003;63(3):311-40.
Behre U, Burow HM, Quinn P, Cree F, Harrison HE. Efficacy of twice-daily dosing of amoxycillin/clavulanate in acute otitis media in children. Infection 1997; 25(3): 163-6. 12. Bottenfield GW, Burch DJ, Hedrick JA, Schaten R, Rowinski CA, Davies JT. Safety and tolerability of a new formulation (90 mg/kg/day divided every 12 h) of amoxicillin/clavulanate (Augmentin) in the empiric treatment of pediatric acute otitis media caused by drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J 1998;17(10):963-8.
|
Pro Otology |
Journal Home Contents Preview Next |