

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 3, No 2—3:86—89 © 2003
All rights reserved. Published by Pro Otology Association
Endoscope-Assisted Microneurosurgery
of Vestibular Schwannomas. Preliminary Results
V. Gerganov, V. Bussarsky, K. Romansky
V. Gerganov, V. Bussarsky, K. Romansky
ABSTRACT
Objective: The aim of the study is to present and discuss our preliminary experience with the application of endoscopy during vestibular schwannoma removal.
Study design: Investigation of the advantages of a neurosurgical technique, applied in a consecutive series of patients.
Settings: Neurosurgical Department at the University Hospital Alexander, Sofia.
Patients: From June 2001 to March 2003, 25 patients with VS have been treated. The endoscope was applied in 8 of them, selected randomly.
Intervention: Retrosigmoid suboccipital approach, supplemented by endoscopic inspection.
Results: In 3 patients with large schwannomas and in 1 with medium sized tumor, the facial nerve location was detected in the initial stages of tumor removal. The internal acoustic canal was inspected endoscopically in all patients and tumor remnant was detected in 1 of them. Unoccluded air cells were not observed. There were no intraoperative complications caused by the use of the endoscope.
Conclusions: The combination of endoscopy and classical microneurosurgery increases the accuracy of the procedure. With the help of the endoscope more microanatomical details can be observed. The facial nerve can be detected endoscopically early in the procedure even in large tumors. The early detection of cranial nerves and vessels helps in the planning of their subsequent dissection and preservation. The risk of leaving tumor remnants or opened air cells in the IAC is obviated.
Key words: Endoscope-assisted microneurosurgery, Vestibular schwannoma, Retrosigmoid approach.
Pro Otology 2-3: 86-89, 2003
Introduction
The application of endoscopy during various neurosurgical procedures reduces their morbidity and mortality. The improved illumination and magnification offered by the endoscope, as well as the possibility “to look around the corner”, lower the risk of damage to critical brain structures. The focal distance of the endoscope is 5- 20 mm and even if higher magnification is used, the visual angle remains wide. The intraoperative use of endoscopes helps to reduce retraction of the brain, cranial nerves and vessels and avoids additional dural or bone resection (1). Pure endoscopic neurosurgery is applied in the treatment of obstructive hydrocephalus, various intraventricular lesions, arachnoid or other parenchymal cysts and pituitary tumors (2). Although the first endoscopic brain surgery was performed more than 100 years ago, the application of the technique continues to broaden.
Endoscope-assisted microsurgery (EAM) combines the advantages of both the classical microneurosurgery and endoscopy. Its application during surgery of vestibular schwannomas (VS) offers the possibility to identify various structures in the cerebellopontine angle (CPA) and their relations. The possibility “to look around the corner” allows the visualization of the fundus of the internal auditory canal (IAC) without incresing the risk of violation to the bony labyrinth. The usage of endoscope as an adjunct to suboccipital retrosigmoid tumor removal avoids some of the disadvantages inherent to the approach- the need of cerebellar retraction, the risk of leaving tumor remnant in the lateral “blind” area of the IAC and the risk of CSF leak through unoccluded opened air cells of the pyramid.
Most of the published articles on the EAM of VS concern the treatment of small or medium sized tumors. The purpose of our study is to present and discuss our early experience with the EAM of large VS.
Materials and methods
Patients
|
|
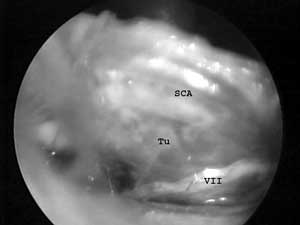
FIG 1. Endoscopic view of the anterior tumor surface after partial removal (Tu - tumor, VII - facial nerve, SCA - superior cerebellar artery). |
From June 2001 to March 2003, 25 patients with VS have been treated at the Department of Neurosurgery, University Alexander Hospital, Sofia. Fifty- six percents of them were female and forty- four– male. Their average age was 49 years, ranging from 17 to 67 years. They were evenly distributed on both sides. NF-II type had 3 patients. Most of the tumors had large size- IV grade according to Samii( s classification. Only one of the tumors was grade III. The surgical approach used in all of the cases was suboccipital retrosigmoid. The total number of the operatins performed is 31. The endoscope was applied in 8 of them, selected randomly. Total tumor removal was achieved in 45% and subtotal - in 27%.
Operative technique
In all cases the retrosigmoid suboccipital approach was used. In the medium-sized tumor the initial inspection with the endoscope provided a panoramic view of the operative field, identification of the structures in the CPA and their relation to the VS. In large schwannomas we performed initialy tumor debulking, then we removed the cranial or the caudal tumor pole. Thus we created a corridor through which we introduced the endoscope and inspected the anterior tumor surface. The identification of the facial nerve guided the subsequent dissection. During the later stages of tumor removal the endoscopic inspection provided us information about the relations of the cranial nerves or vessels to the remaining tumor. After the drilling of the posterior wall of the IAC and the removal of the intrameatal tumor portion, the endoscope was used to look for tumor remnants or opened air cells. At the end of the procedure the anatomic preservation of the cranial nerves and the radicality of tumor removal were documented endoscopically.
Results
The endoscopic inspection of the anterior tumor surface allowed early identification of the facial nerve in 3 patients with large sized VS. In the patient with NF-II, whose VS was grade III and had two smaller schwannomas of the glossopharyngeal nerve, with the endoscope we inspected the CPA at the beginning of the procedure, identified the facial nerve and acquired a detailed view of the caudal nerve schwannomas. In one patient tumor remnant in the lateral part of the IAC, that was not visualized with the microscope, was detected. Opened and unoccluded air cells were not found (FIG. 1, FIG. 2A, FIG. 2B).
Use of the endoscope added 20 minutes, on average, to the total operative time. Procedure-related morbidity was not observed.
Discussion
The application of endoscopy in neurosurgery began in the first decades of the XXth century. At that time Lespinasse and Dandy used the endoscope for the treatment of hydrocephalus. In the 1970s T. Fukushima developed a motorized flexible endoscope and inspected the structures of the CPA (3). Since the end of 1980s, with the elaboration of more perfect endoscopic techniques - new light sources, new instruments, chip video cameras and the miniuaturization of the endoscope, the field of its application is constantly broadening (2). The technique is applied for various skull base lesions - aneurysms, pituitary adenomas, tumors and nerve - compression syndromes. The first articles concerning the use of endoscope in conjunction with an operating microscope during surgery of VS were published in 1990s (4-10).
The treatment of large vestibular schwannomas is surgical, either with the suboccipital retrosigmoid or the translabyrinhtine approach. The retrosigmoid approach is preffered by us, as well as by most neurosurgeons (11-13,15). Its disadvantages have been widely discussed in the literature - need to retract the cerebellar hemisphere, poor visualization of the lateral part of the IAC without violating the labyrinth and increased risk of CSF leak through opened air cells in the region of the drilled IAC (12,14,15). The approach offers a panoramic view of the CPA and the application of endoscopy permits detailed visualization of microanatomical details. The necessity of cerebellar retraction is overcomed by opening and draining the lateral cerebellopontine cistern and by the use of endoscope. Early idenification of cranial nerves and vessels helps to plan their subsequent dissection and preservation (16).
Most frequently the facial nerve in the CPA is located on the anterior tumor surface- on its middle - 39,8% or anterior superior third - 32,7% (17). Consequently, it could be visualized only in the later stages of tumor removal, which increases the risk of its iatrogenic injury. In cases of small or medium sized VS, J. Magnan inspects the anterior tumor surface by introducing the endoscope either superior or inferior to the tumor (18,19). This permits an early identification of the facial nerve in its medial part. In cases of large VS - grade IV, this technique is not applicable. Our approach in such cases is to perform a partial removal of the cranial or caudal tumor poles. Through these surgically created corridors, opposed to the anatomic corridors used by J. Magnan, we inspect the anterior tumor surface. In 3 cases with grade IV and 1 case of grade III VS we could identify the facial nerve running on the anterior tumor surface. Tumor propagation predominately in the direction of the tentorial incisura or to the caudal cranial nerves, was an obstacle to that strategy. The introduction of the endoscope in such cases would endanger the integrity of the surrounding structures.
During the later stages the endoscope was used to inspect and control tumor dissection and removal. It offered better control of the opposite to the surgeon side of the tumor and provided information about the location of the cranial nerves and vessels hidden by the lesion. Especially helpful for us was the identification of the abducent nerve, which is frequently distorted by large schwannomas.
The opening of the posterior wall of the IAC is an essential step of the total tumor removal. However, the most lateral 2-3mm of the canal cannot be examined directly without violating the integrity of the bony labyrinth (20). This increases the risk of leaving residual tumor in that “blind part” (21,22). Some authors point out that the recurrences originate most frequently from that region (23). The possibility “to look around the corner” with the endoscope permits a precise inspection of the whole IAC. After the transverse crest in the fundus of the canal has been exposed, the cochlear and facial nerve can be identified with certainty (20). If residual tumor is found it could be removed under endoscopic or microscopic control. Such a residual tumor, after presumed total tumor removal, is found in 0 - 17,6% (20,24,25). We found tumor remnants in 1 case - 12,5%. This high percentage- higher than the typical for the approach, could be explained by an overrelience on the method. G. Fries and A. Perneczky suggest that the endoscope should be used not only as “a modern mirror” (1). They inspect with the endoscope the whole IAC without drilling its posterior wall and thus reduce the associated morbidity.
The opening of the IAC increases the risk of developing CSF leaks (26,27). The endoscope allows the precise identification and obliteration of opened air cells (28,29). Wackym P, et al., (30) detected such unobliterated air cells in 51 of their series of 108 cases. The endoscopic inspection of the IAC in our patients did not reveal any such cases.
In contrast to intraventricular endoscopy, in the EAM of the skull base the surgeon must navigate through a complex lattice of cranial nerves and vessels (1). During the endoscope- assisted dissection the instruments are introduced and manipulated not through the shaft of the endoscope but outside it. These increases the risk of damage to surrounding structures and determines the more steep learning curve (31). Other disadvantages of the technique are the blood soiling of the endoscopic tip, the lack of appropriate instruments, the lack of control of the surgical field around the proximal part of the endoscope, the two- dimensional image and the potential thermal injury to the nerve structures (24,30). However the latest technical developments and the increasing experience with the EAM, help to overcome these problems.
Conclusion
The EAM allows an early identification of the anatomical structures around the VS - in the IAC and in the CPA. By creating corridors superior or inferior to the tumor, its anterior surface could be inspected even in cases of large schwannomas. The early identification of the facial nerve permits an accurate planning of subsequent tumor removal and its anatomic preservation. The endoscope offers more detailed information than that acquired with the operating microscope. It permits visualization of the whole IAC in the search of tumor remnants or opened air cells without endangering the integrity of the bony labyrinth.
REFERENCES
Fries G., Perneczky A. Endoscope- assisted brain surgery: Part 2- Analysis of 380 procedures. Neurosurgery 1998;42:226-32.
Schroeder HW, Gaab MR. Intracranial endoscopy. Neurosurg Focus 1999;6(4):Article 1,
Matula C, Tschabitscher M, Day JD, et al. Endoscopically assisted microneurosurgery. Acta Neurochir 1995;134:190-5.
Magnan J, Chays A, Caces F, et al. Contribution of endoscopy of the cerebellopontine angle by retrosigmoid approach. Neuroma and vasculo-nervous compression. Ann Otolaryngol Chir Cervicofac 1993;110:259-65.
Magnan J, Chays A, Cohen JM, et al. Endoscopy of the cerebellopontine angle. Laryngol Otol Rhinol 1995;116:115-8.
Magnan J, Chays A, Lepetre C, et al. Surgical perspectives of endoscopy of the cerebellopontine angle. Am J Otol 1994;15:366-70.
Rosenberg SI. Endoscopic otologic surgery. Otolaryngol Clin North Am 1996;29:291-300.
Rosenberg SI, Silverstein H, Willcox TO, Gordon MA. Endoscopy in otology and neurotology. Am J Otol 1994;15:168-72.
McKennan KX. Endoscopy of the internal auditory canal during hearing conservation acoustic tumor surgery. Am J Otol 1993;14:259-62.
O’Donoghue GM, O’Flynn P: Endoscopic anatomy of the cerebellopontine angle. Am J Otology 1993;14:122-5.
Yanargil MG. Management of Accoustic Neuromas, in: Microneurosurgery Vol.IV B, Thieme Verlag, 1996, Sect.2A, Ch.6:100-123.
Samii M, Matthies C. Management of 1 000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 1997;40: 11-21.
Ojemann RG. Retrosigmoid approach to acoustic neuroma /vestibular schwannoma/. Neurosurgery 2001;48:553-8.
Irving RM, Jackler KR, Pitts LH. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J.Neurosurg 1998;88:840-5.
Tonn JC, Schlake HP, Goldbrunner R, et al. Acoustic neuroma surgery as an interdisciplinary approach: a neurosurgical series of 508 patients. J Neurol Neurosurg Psychiatry 2000;69:161-6.
Goksu N, Bayazit Y, Kemaloglu Y. Endoscopy of the posterior fossa and dissection of acoustic neuroma. J Neurosurg 1999;91:776-80.
Sampath P, Rini D, Long DM. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas /acoustic neuromas/: a retrospective study of 1 006 consecutive cases. J Neurosurg 2000;92:70-8.
Magnan J, Barbieri M, Mora R, et al. Retrosigmoid approach for small and medium-sized acoustic neuromas. Otol Neurotol 2002;23: 141-5.
Magnan J. Retrosigmoid approach for acoustic neuroma removal. Pro Otology 2001;3:103-7.
Tatagiba M., Matthies C., Samii M. Microendoscopy of the internal auditory canal in vestibular schwannoma surgery. Neurosurgery 1996;38:737-40.
Schessel D, Nedzelski J, Kand E, Rowed D. Recurrence rate of acoustic neuroma in hearing preservation surgery. Am J Otol 1992;13:231-4.
Mazzoni A., Calabrese V., Moschini L. Residual and recurrent acoustic neuroma in hearing preservation procedures: Neuroradiological and surgical findings. Skull base Surgery 1996;6:105-12.
Thedinger BS, WhittakeK, Luetje CM. Recurrent acoustic tumor after a suboccipital removal. Neurosurgery 1991;29:681-7.
Wackym PA, King WA, Poe DS, et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope 1999;109:1193-201.
Wackym PA, King WA, Weisz DJ. Vascular compression of the cochlear nerve identified by endoscopy during acoustic neuroma surgery. Otolaryngol Head Neck Surg 1999;120:535-6.
Brennan JW, Rowed DW, Nedzelski JM, Chen JM. Cerebrospinal leak after acoustic neuroma surgery: influence of timor size and surgical appproach on incidence and response to treatment. J Neurosurg 2001;94:9-17.
Hoffman RA. Cerebrospinal fluid leak following acoustic neuroma removal. Laryngioscope 1994;104:40-58.
Jennings CR, O’Donoghue GM. Posterior fossa endoscopy. J Laryngol Otol 1998;112:227-9.
Valtonen HJ, Poe DS, Heilman CB, Tarlov EC. Endoscopically assisted prevention of cerebrospinal fluid leak in suboccipital acoustic neuroma surgery. Am J Otol 1997;18:381-5.
Wackym PA, King WA, Meyer GA, Poe DS. Endoscopy in neuro-otologic surgery. Otolaryngol Clin North Am 2002;35:297-323.
King WA, Wackym PA. Endoscope-assisted surgery for acoustic neuromas (vestibular schwannomas): early experience using the rigid Hopkins telescope. Neurosurgery 1999;44:1095-100.
|
Pro Otology |
Journal Home Contents Preview Next |