

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 3, No 2—3:94—98 © 2003
All rights reserved. Published by Pro Otology Association
Morphological Changes of Auditory Ossicles in Experimental Chronic Otitis Media. X-Ray Micro-analysis and Sem Study
Milan Stankovic
ORL Clinic, Medical Faculty, Nis, Serbia
ABSTRACT
Objective: To study morphological changes and osteoclastic osteolysis of auditory ossicles in chronic otitis media
Material and Methods: Pseudomonas aeruginosa (0,1 ml with 4x10-8 bacteria/ml) was inoculated through bulla tympanica in Wistar rats.
Patients: Ten animals served as control, and 30 rats for 15, 60 and 120 days of experiment were used.
Intervention: X-ray microanalysis and scanning electron micro-scopy (SEM) of auditory ossicles were done.
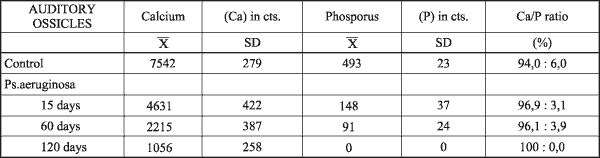
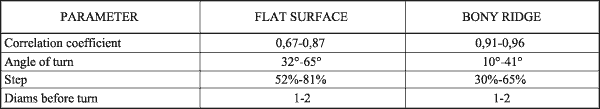
Results: Progressive decrease of calcium, increase of calcium/phosphate ratio and increase of organic material was found. Osteoclastic osteolysis had character of multiple destruction of bone surface with side turns after 12 steps, at angle 10° to 65°, and 30% to 81% from the previous erosion.
Conclusions: Osteolysis is influenced by the characteristics of osteoclasts and bone. Osteoclasts have persistent random movements with translocations.
Key words: Auditory ossicles, Otitis media, Pseudomonas aeruginosa, X-ray, Scanning electron microscopy
Pro Otology 2-3: 94-98, 2003
INTRODUCTION
Structural changes of auditory ossicles in chronic otitis media are important for reconstructive microsurgery of diseased middle ear. Exact assessment of potential applicability of more or less damaged auditory ossicles for tympanoplasties is limited by the possibility to define their morphological alterations under the operation microscope. Potential value of scanning electron microscopy (SEM) for analyses of bone surface changes in different pathological con-ditions was reported. 1-4 Some results of X-ray microanalysis of auditory ossicles in chro-nic otitis media were published (5-7). However, comparative macroscopic, SEM, X-ray micro-ana-lysis, as well as histologic studies of damaged auditory ossicles in chronic otitis media are rare.
Though experimental models can not be directly applied to human conditions, they can define the action and significance of certain parameter. In this way experiment can imply on some facts that are valuable for clinical practice (8-11).
The aim of this experimental study was to analyse the development and course of macro-scopic, SEM and mineral content changes of auditory ossicles in chronic otitis media. Histo-morphometric and enzyme histochemic alterations will be reported in the separate paper.
MATERIAL AND METHODS
Experimental procedure was done on 40 male Wistar rats, nine months old with body weight 180 ( 20 g. Ten animals served as control. Thirty animals for 15, 60 and 120 days of experi-mental period were used. Otoscopy and tympanocenthesis were made before the start of pro-ce-dure to exclude the pathological process in middle ear. Chronic otitis media was induced with hospital type of Pseudomonas aeruginosa that was pathogenic, virulent and resistant to therapy. The culture of Ps. aeruginosa was inoculated in middle ear through a dorsal approach through a burr hole.
All the procedures were done under intraperitoneal pentobarbital (Nembutal Sodium-Abbott) anaesthesia with 35 mg/kg. The animals were kept in standard experimental conditions. After the termination of experiment the animals were killed by pentobarbital overdose and decapi-ta-tion. Microbiologic analysis from the middle ears confirmed Ps. aeruginosa colonisation in all the samples.
Macroscopic study was made on microsurgically separated auditory ossicles under the operation microscope CAPS SOM 12. Shape, diameter and ossicular surface were analysed.
For X-ray microanalysis auditory ossicles were fixed by immersion in a 2% solution of glutaraldehyde (in phosphate buffer at pH 7,4) for 2 hours. They were dehydrated in acetone and dried by the critical point method and examined without coating in JEOL JSM 5300 SEM microscope with X-ray detector LINK QX 2000. The analysis conditions were: counting time 100 s, magnification x1500.
For SEM study auditory ossicles were after X-ray microanalysis coated with gold on JFC 100 Ion sputter. Magnification from 15 to 6000 times was used on the same SEM microscope.
|
|
|
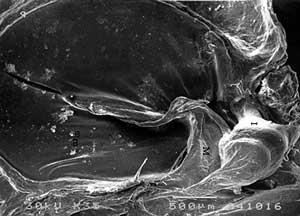
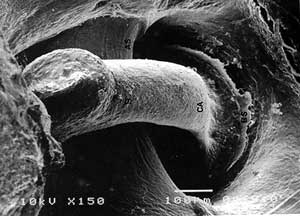
FIG 1. SEM analysis of control auditory ossicles. a) M = malleus, I = incus, TM = tympanic membrane. b) S = stapes, CA = anterior crus, BS = stapedial basis, AS = a.stapedialis. |
|
RESULTS
1. Control group
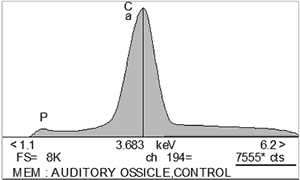
Auditory ossicles of rat resembled human, except for few differences. Malleus was here the biggest ossicle with thin bony lamella attached to head and collum. The ratio between the length of manubrium mallei and long process of incus was 2,4:1 (FIG. 1).X-ray microanalysis gave average 7542 counts for calcium (Ca), and 483 counts for phosphorus (P) (FIG. 2).
Under SEM auditory ossicles had flat epithelium, thin periosteum and flat surface (FIG. 1).
2. Ps. aeruginosa otitis media
Under the operation microscope the presence of auditory ossicles, defect of certain part and character of damage was visible. More precise details of osteolysis were not clear.
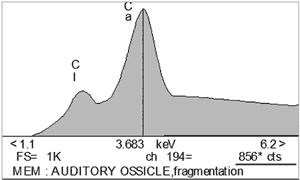
X-ray microanalysis indicated on progressive decrease of Ca level, accompanied by slight increase of Ca/P ratio, and increase of organic material, mainly containing chlorine (Cl). Cl was more prominent than P in granulations over ossicles at the end of experiment (FIG. 2, Table 1).
|
|
|
FIG 2. X-ray microanalysis of auditory ossicles in chronic otitis caused by Ps. aeruginosa (in counts). a) control auditory ossicle with 7555 cts for Ca (upper part), b) fragmentation of ossicles with 856 cts for Ca, depletion of Ca and P, appearance of Cl (lower part). |
|
| Table 1. Semiquantitative X ray microanalysis (in counts) of auditory ossicles in Ps. aeruginosa otitis media. |
|
After removal of granulations around ossicles SEM analysis indicated on different degree of surface lysis. Irregular borders of these osteoclastic osteolyses were in contact with the unchanged bony surface. Prolonged pathological process destroyed normal collagenous struc-ture and invaded deeper bony structures. The bottom of osteons distant from resorption had regular, compact fibers.
Prolonged inflammatory process (120 days) caused marked diminution of areas with unchanged fibers and with mineralization characteristic for resting zones. Serrated resorptive borders of ossicular fragments occupied the most of surface and invaded vascular channels and osteocytic lacunas. There were neither external bony deposits (appositional growth), nor perivascular osteogenesis. The same changes were found for all three ossicles.
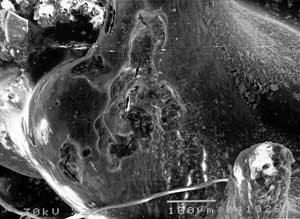
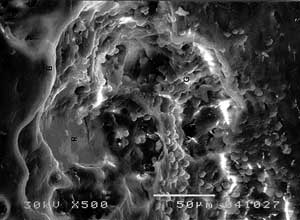
Certain articular parts were degeneratively affected with partly uncovered collagenous fibers and irregular deposits with diameter up to 250 x 45 µm. Osteoclastic movements across ossicu-lar surface made multiple lytic areas that partly folded over each other and had mainly linear course. Osteoclasts often followed the axis of collagen orientation at the surface. Ossicular parts with compact, parallel arrangement of fibers showed resorption with nearly strict linear propagation. Osteoclastic lysis of flat, wider bony surfaces had occasional branching and side turnings, or translocations (FIG. 3).
|
|
|
FIG 3. SEM of auditory ossicles in Ps. aeruginosa otitis media. a) osteoclastic tracs (→) and resorptive areas, b) detail of previous picture with G = granulations, V = vascular channel, R = resorption, B = unchanged bone. |
|
| Table 2. Character of osteoclastic osteolysis of auditory ossicles in Ps. aeruginosa otitis. |
|
Reconstruction of osteoclastic traces indicated that correlation coefficient of osteoclastic movements varied from 0,6 to 0,96. This depended on orientation of collagenous fibers. Resorp-tion moved in steps with side turning after one to two steps, with angle 10° to 65° from the axis of movement. Individual step amounted 30% to 81% from the previous erosion (FIG. 4, Table 2).
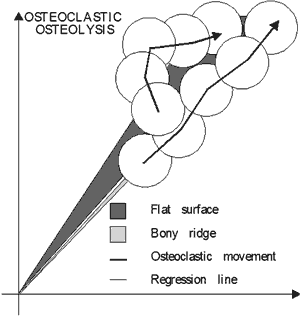
|
|
FIG 4. Osteoclastic osteolysis in steps (→) on flat surfaces and bony ridges. Shaded area represents variation of regression line of osteoclastis movements shown as cos α. |
DISCUSSION
Numerous experimental models for production of chronic otitis media were developped (8-11). Inoculation of Ps. aeruginosa was proven to be one of the best types of experimental osteomyelitis. This bacteria is one of the most frequent in chronic otitis, as well as part of secondary flora after acute inflammation. Virulence of Ps. aeruginosa is attributed to local formation, proliferation, invasion, production of disease and release of toxic materials. Such infection is characterised by necrotic properties, chronicity and resistance on antimicrobic agents 12-14). Thus, investigation of Ps. aeruginosa otitis media is important for determination of extent, character and severity of ossicular involvement and for better surgical therapy.
Macroscopic and SEM studies of auditory ossicles in Ps. aeruginosa otitis have confirmed that judgement of their status under the operation microscope is insufficiently reliable. Some zo-nes with macroscopically regular surface had occasional osteolysis as seen under SEM (1,2,16).
Mendel et al. (1989) using polytomograms and chemical study of damaged incudes in chronic otitis media found normal mineral content of bone near osteolytic part. They concluded that the rest of ossicle is not demineralized (5). Using X-ray microanalysis (Savi et al., 1987) have confirmed advanced decalcination of auditory ossicles in chronic otitis (6). Our results have confirmed significant osteolysis and demineralization caused by inflammation. Undamaged surface is without mineral alteration, but even small osteolytic areas are decalcified.
SEM analyse of bony changes during resorption is reliable. Bone is mineralised, so it is less distorted during preparation. Bone growths appositionally and resorption of bone is also surface phenomenon. SEM significantly contributes to knowledge of bony changes in chronic otitis by adding the third dimension. It enables explanation of mechanism and course of osteolysis. When resorption of bone is minimal its presence is the best detected by surface searching, rather than using histology (14,16).
Regularity of osteoclastic movements across ossicular surface was here verified. As osteoclasts follow the axis of collagen orientation their movements are determined both by the properties of osteoclasts and by bony structure. However, movements of osteoclasts and bone resorptive mechanism seems to be separately controlled actions. The adherence and action of osteoclasts on bone are affected by the chemistry of the material and it’s reaction with components in the culture medium. Computer analysis of osteoclastic movements resembles the persistent random walk, rather than a pure random walk (17,20).
In vitro and in vivo studies have explained some steps in bone resorption. In the beginning narrow demineralised belt can be seen. Osteoclastic secretory products, the speed of resorp-tion and constitution of matrix are influential on this process. Osteoblastic osteolysis before the action of osteoclasts was not proven. Resorptive process of bone is further increased by the action of other inflammatory cells. The correlation of intensity and duration of pathological process and bony lysis in experimental otitis was found (1,2,4,20).
It can be concluded that progressive demineralisation and osteolysis of all three auditory ossicles are found in Ps. aeruginosa otitis media. SEM analyses showed universal surface osteo-clastic osteolysis which is determined by the characteristics of osteoclasts and bone. Osteoclast exhibit persistent random movements with occasional translocations.
REFERENCES
Boyde A, Ali NN, Jones SJ. Optical and Scanning Electron Microscopy in the Single Osteoclast Resorption Assay. Scanning Electron Microscopy 1985;3:1259-71.
Boyde A, Maconnackie E, Reid SA, et al. Scanning Electron Microscopy in Bone Pathology. Rewiev of Methods, Potential and Applications. Scanning Electron Microscopy. 1986;4:1537-54.
Marotti G, Muglia MA, Zaffe D. A SEM Study of Osteocyte Orientation in Alternately Structured Osteons. Bone 1985;6:331-4.
Jones SS, Boyde A, Ali NN. The Interface in Cells and their Matrices in Mineralized Tissues. A Rewiev 1982;4:1555-69.
Mendel L, Bagger Sjöback D, Haverling M, Hjerpe A. The Mineral Content of the Middle Ear Ossicles. A Radiologic and Chemical Study of Normal and Diseased Ossicles. In: Proceedings of III International Conference on Cholesteatoma and Mastoid Surgery, Copenhagen. Kugler & Ghedini. 1989;731-5.
Savi D, Herak R, Djeri D. X-Ray Diffraction Analysis of the Auditory Ossicles in Chronic Otitis. Acta Otolaryngol. 1987;104:125-9.
7. Sanchez-Fernandez JM, Rivera JM, Zubillaga JA. A Microanalysis Study of Human Auditory Ossicles. New Dimensions in Otorhinolaryngology Head and Neck Surgery, Vol 2. Else-vier Science Publishers BV. 1985;161-2.Lewis DM, Schram JL, Maeda SJ, Lim DJ. Experimental Otitis Media in Chinchillas. Ann Otol 1980;68:334-40.
Masaki M, Wright CG, Lee DH, Meyerhoff WL. Experimental Cholesteatoma. Acta Otolaryngol (Stockh). 1989;108:113-21.
Ohashi Y, Nakai Y, Kihara S, Ikeoka H. Effects of Staphylococcus Aureus on the Cilliary Activity in the Middle Ear Lining. Ann Otol Rhinol Laryngol 1987;96:225-8.
Nonomura N, Giebink GS, Juhn SK, et al. Patophysiology of Streptococcus Pneumoniae Otitis Media : Kinetics of the Middle Ear Biochemical and Cytologic Host Responses. Ann Otol Rhinol Otol 1991;110:236-43.
Norden CW. Animal Models of Osteomyelitis. Ann Otol Rhinol Laryngol 1988;131:18-9.
Pollack M. Special Role of Pseudomonas Aeruginosa in Chronic Suppurative Otitis Media. Ann Otol Rhinol Laryngol 1988;131:10-3.
Davis SD. Interaction of Pseudomonas and the Host. Ann Otol Rhinol Laryngol 1988;131:13-5.
Hellström J, Salen B, Stenfors LE. Anatomy of the Rat Middle Ear. Acta Anat 1982;112:346-52.
Chole RA. Osteoclasts in Chronic Otitis Media and Otosclerosis. Ann Otol Rhinol Laryngol 1988;97:661-6.
Albrecht-Buchler G. The Angular Distribution of Directional Changes of Guided 3T3 Cells. J Cell Biol 1979;80:53-60.
Enteneur U, Schliwa M. Persistent, Directional Motility of Cells and Cytoplasmic Fragments in the Absence of Microtubules. Nature 1984;310:58-61.
Smyth AL, Boyde A, Jones SJ. Computer Stimulation of Osteoclastic Motility During Resorptive Episodes in Vitro. Calcif Tiss Int 1985;38:18-20.
Krempien B, Klimpel L. Scanning Electron Microscopical Studies of resorbing Surfaces. In: Lee WSS, Parfitt AM, eds. Bone Histomorphometry. Paris: Armour Montagy, 1981:45.
|
Pro Otology |
Journal Home Contents Preview Next |