

|
Journal Home Contents Preview Next |
Pro Otology
Balkan Journal of Otology & Neuro-Otology, Vol. 4, No 2-3:107—109 © 2004
All rights reserved. Published by Pro Otology Association
Modiolar Implantation –
a New Alternative to Cochlear Implantation
*K. Assenova, †St. Stoyanov, *T. Karchev, *†V. Ovcharov
*Medical University Sofia, ENT Department, Bulgaria
†Ministry of Interior – Medical Institute, ENT Department, Sofia, Bulgaria
*†Medical University Sofia, Department of Anatomy and Histology, Sofia, Bulgaria
ABSTRACT
Objective:The aim of our study was to prove that implantation of an electrode into the modiolus is technically possible without major anatomic damages.
Methods: 10 dissections of human temporal bones were carried out. A plastic electrode was introduced in the modiolus.
Results: It was found that the appropriate surgical approach to the modiolus is through middle cranial fossa. The cupula of the cohlea is located medially and anteriorly to ganglion geniculli. As landmark serves the greater petrosal nerve. The nerve is elevated, the bone is drilled from its sulcus and the cupula is found at about 2mm depth. After entering the cupula, the top of the modiolus is visualized. A canal along canalis longitudinalis modioli should be made by a thin probe, which is introduced with gentle pressure due to the very soft modiolar tissue. Entering of the first 2 mm in the internal auditory canal does not harm the still separated nerve fibers. Histological studies did not show significant impairment of modiolar anatomic structures.
Conclusion: We suppose that the implantation of an electrode in the modiolus could be an alternative to standard cochlear implantation and may even be preferable in cases with chronic ear inflammation and cochlear ossification. A modiolar electrode with a length of about 7 - 8 mm would not damage the auditory nerve trunk.
Keywords: Modiolus, Implantation, Otosurgery.
Pro Otology 2-3:107—109, 2004
Introduction
Despite of their continuous refinement, cochlear implants still have numerous limitations in their use. They are contraindicated in patients with middle or internal ear malformations, in cases after radical mastoidectomy, severe cochlear ossification or chronic otitis media. Although the use of an implant leads to significant improvement of speech perception, the sound sensation is far from physiological. To some extent this is caused by the limitation in time and frequency coding due to the relatively great distance between the electrode and the ganglion cells, which leads to the demand of significant stimulation current levels and lower stimulation selectivity.
The modern trends in cochlear implantation are for development of a fully implantable device which has the highest possible coding capabilities. This requires minimal energy consumption, which may be achieved by improvement both of the speech processor and electrode design and optimal electrode placement as near as possible to the spiral ganglion cells (1).
The significant stimulation current levels remain one of the main problems which modern cochlear implantation surgery has to solve. A step ahead in this direction is the introduction of the so called hugging (perimodiolar) electrode (Nucleus 24 Contour, MedEl Pulsar) which has significantly lower threshold and comfortable levels. That benefit is a result from placing the electrode array closer to the spiral ganglion cells (2,3).
The interface between the electrode and cochlear nerve is a key area in which improvements of cochlear implantation might be effected. Notable decrease in stimulation threshold currents and power consumption could be achieved by an electrode implanted directly into the cochlear nerve. The use of penetrating electrode is not a new idea. Simmons (4) reported on cochlear nerve implantation, performed with six stainless steel electrodes wound around each other and insulated to the tips.
Hillman and al. (5) developed an array of silicon-based electrode needles so called the Utah Slanted Electrode Array. This array with a three-dimensional penetrating electrode architecture could achieve more focal stimulation than a scalar array and, as a result, greater frequency selectivity. The cochlear nerve was exposed using a transcochlear approach. This technique increases the risk of injuring the nerve during surgery. The possibility of CSF leakage and risk of meningitis are other possible complications.
|
|
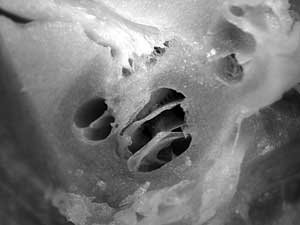
FIG. 3. A longitudinal section through the cochlea and internal auditory meatus is performed. The basal, middle and apical turns of the cochlea are shown. The separate nerve fibers join to form auditory nerve trunk about 2 mm after emerging from tractus spiralis foraminosus. |
Ho et al. (6) state that a modiolar return electrode significantly increases the current flow across spiral ganglion cells into the modiolus, and may decrease the cochlear implant’s power requirements. They study the effects of return electrode positioning on current flow in the modiolus in a Plexiglas model of the cochlea. Results of model measurements are confirmed by measurements in the modiolus of human temporal bones.
Colletti et al. (7,8) are the first authors that use middle cranial fossa approach for cochlear implantation. The electrode is introduced into the basal turn of the cochlea. One of the advantages of this method is that middle ear is bypassed and in this way the risk of infection – lowered.
3D computer-assisted navigation can be an essential tool for preoperative planning, assessment of target structures and intraoperative orientation by definition of anatomical relationships important for cochlear implantation. This technology can be useful for lowering intraoperative risks and optimization of microsurgical manipulations (9).
Methods
Our experiment was carried out on ten human cadaver temporal bones. With adequate exposure of the middle cranial fossa floor and after identification of the greater superficial petrous nerve and arcuate eminence, drilling was started at the bony angle between ganglion geniculi and greater superficial petrous nerve. After cochleostomy was performed, the top of the modiolus, surrounded by apical cochlear coil was visualized (FIG. 1). A thin probe was inserted through the central portion of the modiolus from top to bottom (FIG. 2). A plastic electrode was then introduced into the canal, produced by the probe. Histological study of the “implanted” bones was performed.
Results and discussion
It is well known that the cochlea is in contact with the anterior petrous wall in its anterosuperior region. The superior edge of the cupula cochleae is situated about 2 mm under and just medially and anteriorly to the ganglion geniculi. As surgical landmarks were used the arcuate eminence and especially the greater superficial petrosal nerve. The disclosure of cupula cochleae sometimes required petrous nerve resection.
The length of modiolar axis is about 5 mm, however the modiolar nerve fibers join to form the auditory nerve 2–3 mm after leaving tractus spiralis foraminosus (FIG. 3). This means that modiolar electrode with a length of about 7 - 8 mm would not damage the auditory nerve trunk.
The bodies of auditory neurons are located into Rosenthal’s canal, spirally wound around the modiolus (FIG. 4). The modiolus itself is a spongiose bone structure with multiple canals containing the axons of ganglion cells.
|
|
The density of innervation, as measured by the presence of neurons, is highest in the upper basal and lower middle coils (10). Sacrifice of nerve fibers and neurons at the apical part of the modiolus would not have serious consequences in voice perception.
Arterial blood supply to the cochlea is maintained by an artery running spirally around the modiolus. Arterioles leave the artery running centrifugally and radiate both over the scala vestibuli and over the spiral lamina, ramifying several times. The spiral capillary systems in the external wall and in the spiral lamina are drained by centripetally radiating collecting venules which empty into one or two veins running spirally around the modiolus (11). This supports our observations that in the central part of the modiolus there are no blood vessels, which could raise bleeding problem during the implantation (FIG. 5).
Conclusions
The structure of petrous bone allows relatively harmless opening of the cochlea through middle cranial fossa.
The superior edge of the cupula cochleae is under and just medially and anteriorly to the ganglion geniculi.
The arcuate eminence and especially the greater superfitial petrosal nerve can serve as reliable surgical landmarks.
A relatively broader cochleostomy facilitates the visualization of modiolar apex, surrounded by the apical cochlear turn.
An electrode with a length of about 7-8 mm could be introduced into the modiolus without impairment of important modiolar structures and trunk of cochlear nerve.
Modiolar implantation via middle cranial fossa approach has the following advantages:
The direct stimulation of ganglion cells’ axons would significantly decrease electric current levels that allow economy of energy.
The stimulation of auditory neurons is achieved by greater selectivity.
3. Implantation of an electrode in the modiolus could be first choice alternative in cases with severe cochlear ossification, cochlear malformations, chronic otitis media or radical mastoidectomy.
REFERENCES
Lenarz T. Cochlear implants: what can be achieved? Am. J. Otol. 1997;6:2-3.
Saunders El., Cohen L, Aschendorff A. et al. Threshold, comfortable level and impedance as a function of electrode-modiolar distance. Ear & Hearing 2002;23:28-40.
Roland JT., Fishman AJ, Alexiades G. Electrode to modiolus proximity: a fluoroscopic and histological analysis. Am. J. Otol. 2000; 21:218-5.
Simmons FB., Epley JM., Lummis RC. et al. Auditory nerve electrical stimulation in man. Science 1965;148:104-6.
Hillman T, Badi AN, Normann RA et al. Cochlear nerve stimulation with a 3-dimensional penetrating electrode array. Otol. Neurotol. 2003;24(5):764-8.
Ho S, Wiet R, Richter C. Modifying cochlear implant design: advantages of placing a return electrode in the modiolus. Otol. Neurotol. 2004;25:497–503.
Colletti V, Fiorino FG., Carner M. Basal turn cochleostomy via the middle fossa route for cochlear implant insertion. Am. J. Otol. 1998;19(6):778-84.
Colletti V, Fiorino FG. New window for cochlear implant insertion. Acta Otolaryngol. 1999;119(2):214-7.
Vogele M, Freysinger W, Bale R. Use of the ISG viewing wand on the temporal bone. A model study. HNO. 1997;45(2):74-80.
Spoendlin H. Innervation densities of the cochlea. Acta otolaryngol. 1972;73:235-48.
Axelsson A. The vascular anatomy of the cochlea in the guinea pig and man. Acta otolaryngol.1968;243:1-134.
|
Pro Otology |
Journal Home Contents Preview Next |